
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 124.0°c. (cwater 118 jig'c, gee 2.03 jig c, g team jig c, molar heat of fusion of ice 6.01 * 10 j/mol; molar heat of vaporization of liquid water 4.07 * 10*j/mol 202 short answer toolbar navigation b i v s e 1 e a a this question will be sent to your instructor for grading

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, bbombard21
Select the atomic models that belong to the same element
Answers: 2

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 1...
Questions in other subjects:







Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

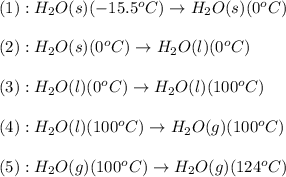
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0213/6323/e4ef0.png)
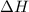 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?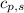 = specific heat of solid water =
= specific heat of solid water = 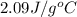
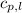 = specific heat of liquid water =
= specific heat of liquid water = 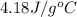
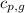 = specific heat of liquid water =
= specific heat of liquid water = 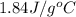
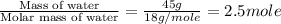
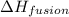 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole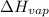 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[45g\times 4.18J/gK\times (0-(-15.5))^oC]+2.5mole\times 6010J/mole+[45g\times 2.09J/gK\times (100-0)^oC]+2.5mole\times 40670J/mole+[45g\times 1.84J/gK\times (124-100)^oC]](/tpl/images/0213/6323/555ef.png)
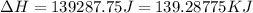 (1 KJ = 1000 J)
(1 KJ = 1000 J)

