
Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(s) → 2 al2o3(s) + 3 mn(s) calculate the enthalpy change of reaction. given: 2al(s) + 3/2 o2(g) →al2o3(s) ah = -1680 kj mol'. mn(s) + o2(g) → mno2(s) ah = -520 kj mol! .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(...
Questions in other subjects:


Mathematics, 20.03.2020 05:00






Mathematics, 20.03.2020 05:01

Mathematics, 20.03.2020 05:01

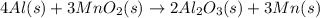





![\Delta H=[n\times \Delta H_1]+[n\times (-\Delta H_2)]](/tpl/images/0213/0280/c45d3.png)
![\Delta H=[2mole\times (-1680kJ/mole)]+[3\times -(-520kJ/mole)]](/tpl/images/0213/0280/514b6.png)



