
Chemistry, 30.08.2019 21:10 Princess14321
The combustion of octane is given in this reaction 2c8h18++18h2 o, identify the limit reagent for this reaction with respect of carbon dioxide given starting mass of 12.85g of octane and 7.46g of oxygen.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
The combustion of octane is given in this reaction 2c8h18++18h2 o, identify the limit reagent for th...
Questions in other subjects:

Mathematics, 22.01.2021 02:20

Health, 22.01.2021 02:20

History, 22.01.2021 02:20


Mathematics, 22.01.2021 02:20

Mathematics, 22.01.2021 02:20



History, 22.01.2021 02:20

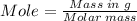

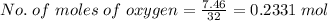
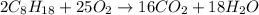
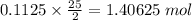 of oxygen.
of oxygen.


