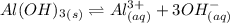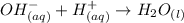Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Consider the equilibrium of al(oh)3 (s) in water. al(oh)3 (s) = a13+ (aq) + 30h" (aq) how is the sol...
Questions in other subjects:

Social Studies, 01.12.2021 05:20

Law, 01.12.2021 05:20

Mathematics, 01.12.2021 05:20


Mathematics, 01.12.2021 05:20



World Languages, 01.12.2021 05:20

Mathematics, 01.12.2021 05:20

Mathematics, 01.12.2021 05:20