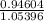Chemistry, 30.08.2019 17:20 brisamauro27
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a solution that is 1.0o m in sodium acetate and 1.00 m in acetic acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 16:50, TrueKing184
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a soluti...
Questions in other subjects:

English, 15.05.2021 06:40


Arts, 15.05.2021 06:40

History, 15.05.2021 06:40

Biology, 15.05.2021 06:40

Mathematics, 15.05.2021 06:40


Mathematics, 15.05.2021 06:40


Mathematics, 15.05.2021 06:40

 value of acetic acid is 4.74. And, relation between pH and
value of acetic acid is 4.74. And, relation between pH and ![\frac{[CH_{3}COOH]}{[CH_{3}COONa]}](/tpl/images/0212/4640/b577a.png)

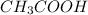 = moles of
= moles of 

![\frac{[CH_{3}COONa]}{[CH_{3}COOH]}](/tpl/images/0212/4640/ccc54.png)