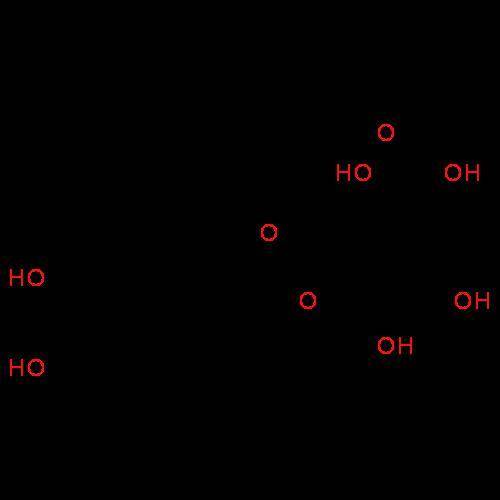
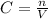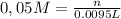Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an indicator solution and titrates with sodium hydroxide. he finds the equivalence point occurs when he's titrated 9.5 ml of 0.05 m sodium hydroxide. approximately how much chlorogenic acid is in the sample? 475 umol ® 480 umol © 500 umol 510 umol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 09:10, amandapill
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 13:30, emmilicious
Malik formed a hypothesis that an increase in atmospheric oxygen levels by 10% would cause red-legged grasshoppers to grow larger than normal. suppose that malik performs an experiment to test his hypothesis. which of these actions would represent a scientific mistake in his experiment? a. he experiments on live grasshoppers instead of preserved ones. b. he focuses on red-legged grasshoppers instead of all kinds of grasshoppers. c. he varies the nitrogen and carbon dioxide levels in the air from one trial to the next. d. he conducts the experiment in a controlled lab setting with a lab partner. e. he measures the mass and length of his specimens at the start of each trial.
Answers: 1

Chemistry, 23.06.2019 19:00, masonvinyard02p83vua
Hjulstrom's diagram plots two curves representing (1) the minimum stream velocity required to erode sediments of varying sizes from the stream bed, and (2) the minimum velocity required to continue to transport sediments of varying sizes. this is a modified version of the diagram, showing only the transport segment. stream competence refers to the heaviest particles a stream can carry. according to hjulstrom's diagram stream competence depends on a) particle size. b) stream velocity. c) stream deposition. d) stream channel shape.
Answers: 2
You know the right answer?
Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an in...
Questions in other subjects:


Biology, 30.04.2021 23:40

Chemistry, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40



Mathematics, 30.04.2021 23:40