

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, JotaroKujo6233
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 01:00, deidaralove90
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

You know the right answer?
Abuffer consists of 0.120 m hno2 and 0.150 m nano2 at 25°c. pka of hno2 is 3.40. a. what is the ph o...
Questions in other subjects:



Biology, 28.02.2020 22:47







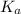 of
of 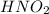 =
= 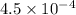 .
.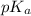 is as follows.
is as follows.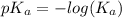
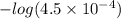
![pK_{a} + log\frac{[conjugate base]}{[acid]}](/tpl/images/0212/3783/ce755.png)
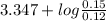
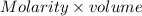

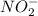 will react with
will react with 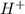 to form
to form 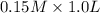
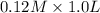
![pK_{a} + log \frac{[conjugate base]}{[acid]}](/tpl/images/0212/3783/4843d.png)


