Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

You know the right answer?
Iron (iii) oxide reacts with carbon monoxide to produce iron metal and carbon dioxide. what mass of...
Questions in other subjects:

Mathematics, 09.07.2021 22:20



Mathematics, 09.07.2021 22:20



Mathematics, 09.07.2021 22:20



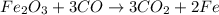
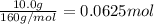
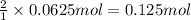 of iron metal.
of iron metal.