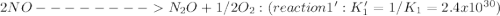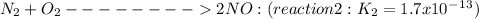Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x 10-13 find the value of the equilibrium constant for the following equilibrium reaction: n2(g) 1/202(g) n20(g) a) 7.0 x 10-44 b) 4.2 x 1017 c) 2.4 x 10-18 d) 1.6 x 109 e) 2.6 x 10-22

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, fendyli2353
Brad and his family like to go camping. they always take a gas lantern with them. the lantern can take fiuels but always puts out the same amount of light. brad has two different kinds of fuel for the lantern. how can he figure out which kind of fuel has more chemical energy that the lantern can turn into light? measure the brightness of the lantern using each kind of fuel by comparing it to the full moon. g measure the volume of the containers of fuel. measure how long the lantern stays lit on a fixed amount of fuel. measure the mass of each container of fuel using a balance scale. answer 12
Answers: 1

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x...
Questions in other subjects:





Mathematics, 30.11.2021 22:50


Engineering, 30.11.2021 22:50


Arts, 30.11.2021 23:00