

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
For a galvanic cell that uses the following two half-reactions, cr 2o 7 2-( aq) 14 h ( aq) 6 e - → 2...
Questions in other subjects:

Mathematics, 08.03.2021 23:00

Chemistry, 08.03.2021 23:00


Chemistry, 08.03.2021 23:00





History, 08.03.2021 23:00

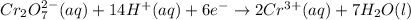
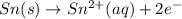
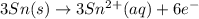
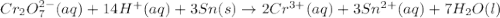
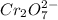 will oxidizes 3 moles of Sn
will oxidizes 3 moles of Sn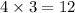 moles of Sn
moles of Sn

