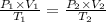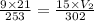Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 06:30, SimplyGenesis762
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2

Chemistry, 23.06.2019 06:40, Taylor73836
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
If a gas is initially at a pressure of 9 atm, a volume of 21 liters, and a temperature of 253 k, and...
Questions in other subjects:




History, 08.09.2020 19:01

Mathematics, 08.09.2020 19:01


Mathematics, 08.09.2020 19:01

History, 08.09.2020 19:01

Mathematics, 08.09.2020 19:01

Business, 08.09.2020 19:01