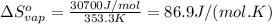Chemistry, 30.08.2019 01:30 veneciaconton347
The enthalpy of vaporization (δh°vap) of benzene is 30.7 kj/mol at its normal boiling point of 353.3 k. what is δs°vap at this temperature? a. 383 j/(mol·k) b. 0.0115 j/(mol·k) c. 86.9 j/(mol·k) d. 0.087 j/(mol·k) e. 11.5 j/(mol·k)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
The enthalpy of vaporization (δh°vap) of benzene is 30.7 kj/mol at its normal boiling point of 353.3...
Questions in other subjects:

English, 15.12.2020 22:00


Mathematics, 15.12.2020 22:00







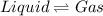
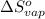 for the reaction, we use the equation:
for the reaction, we use the equation: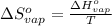
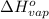 = standard enthalpy change of vaporization = 30.7 kJ/mol = 30700 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard enthalpy change of vaporization = 30.7 kJ/mol = 30700 J/mol (Conversion factor: 1 kJ = 1000 J)