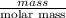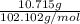Chemistry, 29.08.2019 19:10 sarmientojose267
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. beakers 1 through 4 contain 2.3, 1.91, 5.985, and 0.52 g of dry potassium nitrate respectively. how many moles of potassium nitrate were recovered after the water evaporated?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. be...
Questions in other subjects:


Health, 06.02.2021 23:10

Health, 06.02.2021 23:10


Mathematics, 06.02.2021 23:10



Mathematics, 06.02.2021 23:10

History, 06.02.2021 23:10

History, 06.02.2021 23:10

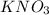 = molar mass of K + molar mass of N + 3 × molar mass of O
= molar mass of K + molar mass of N + 3 × molar mass of O