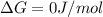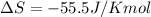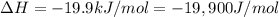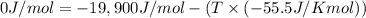Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
For a given reaction, ah = -19.9 kj/mol and as = -55.5 j/k/mol. the reaction will have ag = 0 at k....
Questions in other subjects:

Mathematics, 02.02.2021 22:20




Health, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20


Advanced Placement (AP), 02.02.2021 22:20


Mathematics, 02.02.2021 22:20

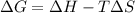
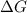 = Change in Gibbs free energy
= Change in Gibbs free energy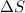 = Change in an entropy
= Change in an entropy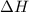 = Enthalpy of reaction
= Enthalpy of reaction