
Chemistry, 28.08.2019 23:30 lilday8230
22 400 ml of oxygen gas contains 6.022 1023 oxygen molecules at 0°c and standard atmospheric pressure. a. how many oxygen molecules are in 0.100 ml of gas? b. how many oxygen molecules are in 1.00 l of gas? c. what is the average space in milliters occupied by one oxygen molecule?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1
You know the right answer?
22 400 ml of oxygen gas contains 6.022 1023 oxygen molecules at 0°c and standard atmospheric pressur...
Questions in other subjects:

Mathematics, 13.04.2021 17:40






Mathematics, 13.04.2021 17:40



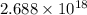
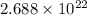
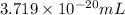
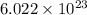 number of oxygen molecules
number of oxygen molecules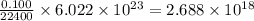 number of oxygen molecules
number of oxygen molecules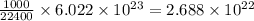 number of oxygen molecules
number of oxygen molecules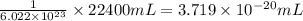 of oxygen gas
of oxygen gas

