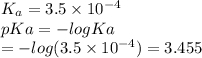Chemistry, 28.08.2019 21:30 Andrewecolt1993
Which of the following acids (listed with ka values) and their conjugate base would form a buffer with a ph of 2.34? a. hf, ka = 3.5 x 10-4 b. c6h5cooh, ka = 6.5 x 10-5 c. hclo2, ka = 1.1 x 10-2 d. hclo, ka = 2.9 x 10-8 e. hio3, ka = 1.7 x 10-1

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 04:31, diamondscott9297
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 05:00, cpcoolestkid4
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius. a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit. a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius. a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Which of the following acids (listed with ka values) and their conjugate base would form a buffer wi...
Questions in other subjects:




Mathematics, 18.01.2021 18:20



Mathematics, 18.01.2021 18:20