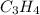Chemistry, 28.08.2019 21:10 heavenwagner
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone and phenol in the chemical industry. combustion of 47.6 mg cumene produces some co2 and 42.8 mg water. the molar mass of cumene is between 115 and 125 g/mol. determine the empirical and molecular formulas.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone a...
Questions in other subjects:

Mathematics, 01.10.2021 17:20

Mathematics, 01.10.2021 17:20

Mathematics, 01.10.2021 17:20

Physics, 01.10.2021 17:20

Law, 01.10.2021 17:20

Mathematics, 01.10.2021 17:20

Biology, 01.10.2021 17:20

English, 01.10.2021 17:20

History, 01.10.2021 17:20

Arts, 01.10.2021 17:20

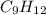
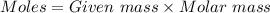
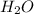 = 42.8/18 = 2.3778×10⁻³ moles
= 42.8/18 = 2.3778×10⁻³ moles