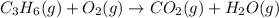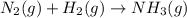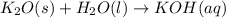Chemistry, 28.08.2019 17:20 mauricio18s
Balance the following equations, and indicate whether they are combination, decomposition, or combustion reactions.1. c3h6(g)+o2(> co2(g)+h2o(g)1a. is it a combination reaction, decomposition reaction, or combustion reaction2. nh4no3(> n2o(g)+h2o(l)2a. is it a combination reaction, decomposition reaction, or combustion reaction3. c5h6o(l)+o2(> co2(g)+h2o(g)3a. is it a combination reaction, decomposition reaction, or combustion reaction4.n2(g)+h2(> nh3(g)? 4a. is it a combination reaction, decomposition reaction, or combustion reaction5. k2o(s)+h2o(> koh(aq)5a. is it a combination reaction, decomposition reaction, or combustion reaction

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, marissastewart533
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Balance the following equations, and indicate whether they are combination, decomposition, or combus...
Questions in other subjects:

History, 29.01.2020 15:48


History, 29.01.2020 15:48

Mathematics, 29.01.2020 15:48


Mathematics, 29.01.2020 15:49



Chemistry, 29.01.2020 15:49