Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Given that δh = −571.6 kj/mol for the reaction 2 h2(g) + o2(g) → 2 h2o(l), calculate δh for these re...
Questions in other subjects:

History, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40




Mathematics, 04.12.2020 18:40


Chemistry, 04.12.2020 18:40

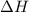 for the reaction is +571.6 kJ/mole.
for the reaction is +571.6 kJ/mole.


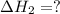
 for the reaction will be:
for the reaction will be: