
Chemistry, 28.08.2019 03:20 cibitoye8627
What element is being oxidized in the following redox reaction? fe 2+(aq) + nh4 +(aq) → fe(s) + no3 -(aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 21:50, isabel81ie
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l. s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2


Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
You know the right answer?
What element is being oxidized in the following redox reaction? fe 2+(aq) + nh4 +(aq) → fe(s) + no3...
Questions in other subjects:


Biology, 19.12.2019 19:31








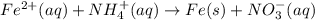
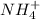 is as follows.
is as follows.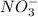 is as follows.
is as follows.

