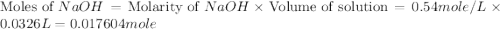Chemistry, 27.08.2019 20:30 alexiaaaa234
In a titration, 4.7 g of an acid (hx) requires 32.6 ml of 0.54 m naoh(aq) for complete reaction. what is the molar mass of the acid? answer in units of g/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
In a titration, 4.7 g of an acid (hx) requires 32.6 ml of 0.54 m naoh(aq) for complete reaction. wha...
Questions in other subjects:

Mathematics, 21.02.2020 02:52

History, 21.02.2020 02:52




Mathematics, 21.02.2020 02:52



World Languages, 21.02.2020 02:52

Mathematics, 21.02.2020 02:52