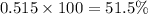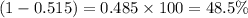The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x, one weighing 59 g/mol, and the other weighing 61 g/mol. what is the relative abundance of each?
possible answers:
50% x-59, 50% x-61
75% x-59, 25% x-61
63% x-59, 37% x-61
51.5% x-59, 48.5% x-61
48.5% x-59, 51.5% x-61

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x...
Questions in other subjects:

Mathematics, 04.02.2021 17:10

Mathematics, 04.02.2021 17:10


Geography, 04.02.2021 17:10

Social Studies, 04.02.2021 17:10

Mathematics, 04.02.2021 17:10

Mathematics, 04.02.2021 17:10


Mathematics, 04.02.2021 17:10

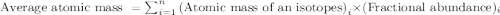 .....(1)
.....(1)![59.97 g/mol=[(59 g/mol\times x)+(61 g/mol\times (1-x))]\\\\x=0.515](/tpl/images/0202/9296/4c349.png)