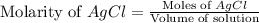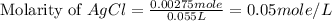Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn't change when the iron(ii) chloride is dissolved in it.
be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nit...
Questions in other subjects:


Chemistry, 10.10.2020 14:01

History, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01

Biology, 10.10.2020 14:01

Biology, 10.10.2020 14:01

Chemistry, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01

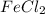 = 0.701 g
= 0.701 g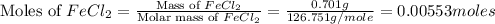
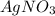 .
.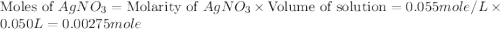
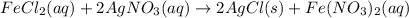
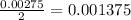 moles of
moles of 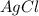 .
.