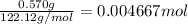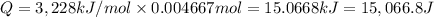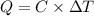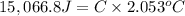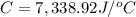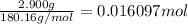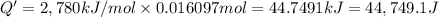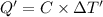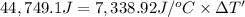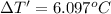Chemistry, 27.08.2019 17:10 aliciajackson26
The combustion of 0.570 g of benzoic acid (δhcomb = 3,228 kj/mol; mw = 122.12 g/mol) in a bomb calorimeter increased the temperature of the calorimeter by 2.053naughtc. the chamber was then emptied and recharged with 2.900 g of glucose (mw = 180.16 g/mol) and excess oxygen. how much did the temperature change from the combustion of the glucose? δhcomb for glucose is 2,780 kj/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1
You know the right answer?
The combustion of 0.570 g of benzoic acid (δhcomb = 3,228 kj/mol; mw = 122.12 g/mol) in a bomb calo...
Questions in other subjects:



Mathematics, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40



Mathematics, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40