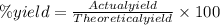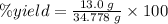Chemistry, 27.08.2019 17:10 hopelesslylost13
In making wine, glucose (c6h12o6) is fermented to produce ethanol (c2h5oh) and carbon dioxide (co2), according to the following reaction. c6h12o6 → 2 c2h5oh + 2 co2 (a) if the fermentation reaction starts with 68.0 g glucose, what is the theoretical yield of ethanol (in grams)? g (b) if 13.0 g ethanol is produced, what is the percent yield of this reaction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
In making wine, glucose (c6h12o6) is fermented to produce ethanol (c2h5oh) and carbon dioxide (co2),...
Questions in other subjects:



Biology, 03.11.2019 00:31


Chemistry, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31

History, 03.11.2019 00:31


Biology, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31