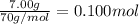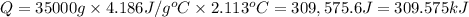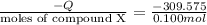Chemistry, 27.08.2019 17:10 sakurauchiha913
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25°c. the temperature of the water is observed to rise by 2.113°c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25°c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. [could you show a step by step way to solve this]

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter conta...
Questions in other subjects:







Mathematics, 22.05.2020 02:13

English, 22.05.2020 02:13