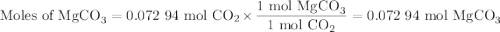Chemistry, 26.08.2019 23:30 chloiesierra29
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g) . after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 17.48 g. assum- ing that only the magnesium carbonate had decomposed, what was the percent of magne- sium carbonate in the original sample? answer in units of %.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2


Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to th...
Questions in other subjects:

English, 15.07.2019 00:30


Mathematics, 15.07.2019 00:30




English, 15.07.2019 00:30

Chemistry, 15.07.2019 00:30


Mathematics, 15.07.2019 00:30