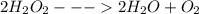Chemistry, 26.08.2019 23:30 hailiemanuel930
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a concentrated hydrogen peroxide solution. hydrogen peroxide decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). manganese(iv) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. what are the coefficients in the balanced chemical equation for this reaction?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a...
Questions in other subjects:

English, 13.07.2019 05:30




Chemistry, 13.07.2019 05:30




Health, 13.07.2019 05:30