
Chemistry, 26.08.2019 22:10 cecelia090
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample of epsom salts is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 02:40, alexandraparava
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample o...
Questions in other subjects:





Mathematics, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31

World Languages, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31

 = 120 g/mol
= 120 g/mol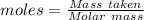
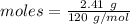

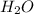 = 18 g/mol
= 18 g/mol

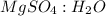 = 0.02 : 0.14 = 1 : 7
= 0.02 : 0.14 = 1 : 7


