
Chemistry, 26.08.2019 19:00 jadenicole908
You analyze two substances in the laboratory and discover that each has the empirical formula ch2o. you can easily see that they are different substances because one is a liquid with a sharp, biting odor and the other is an odorless, crystalline solid. how can you account for the fact that both have the same empirical formula?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
You analyze two substances in the laboratory and discover that each has the empirical formula ch2o....
Questions in other subjects:

Mathematics, 10.12.2020 21:30






History, 10.12.2020 21:30



English, 10.12.2020 21:30

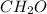
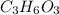 in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.
in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.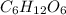 in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.
in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.

