
Chemistry, 26.08.2019 18:00 juan01sebastian00
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen molecules and six hydrogen molecules in a closed container. assuming the reaction goes to completion, what will the final product mixture be?
a. number of nh3 molecules
b. number of n2 molecules
c. number of h2 molecules
which of the following equations best represents this reaction?
a. 42 n2 + 6 h2 4 nh3
b. 6 n2 + 6 h2 4 nh3 + 4 n2
c. n + 3 h2 nh3
d. n2 + 3 h2 2 nh3
e. n2 + h2 nh3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, mildredelizam
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen mole...
Questions in other subjects:

Mathematics, 13.10.2020 15:01



English, 13.10.2020 15:01


Biology, 13.10.2020 15:01

History, 13.10.2020 15:01



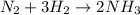
 react completely with 1 molecule of
react completely with 1 molecule of 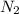 and produce 2 molecules of
and produce 2 molecules of 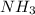 .
.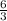 or 2 molecules of
or 2 molecules of  or 4 molecules of
or 4 molecules of 

