***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqu...

Chemistry, 25.08.2019 21:10 Andrebutrus
***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqueous hydrochloric acid in a calorimeter at an initial temperature of 23.0 degrees celsius, what is the enthalpy change of this reaction if the final temperature reached in the calorimeter is 25.5 degrees celsius?
naoh + hcl yields nacl + h2o

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

You know the right answer?
Questions in other subjects:



Arts, 14.11.2019 02:31

Chemistry, 14.11.2019 02:31



Biology, 14.11.2019 02:31



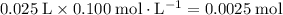 of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.
of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.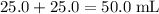 .
.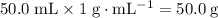 .
.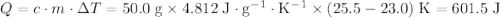
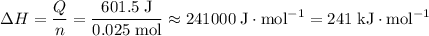 .
.


