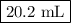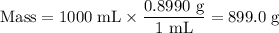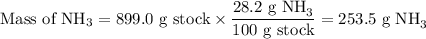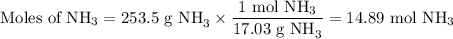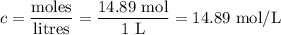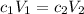Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, peaceouthjkdrb2398
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1
You know the right answer?
Astock solution is 28.2 percent ammonia (nh3) by mass, and the solution has a density of 0.8990 gram...
Questions in other subjects:

Mathematics, 30.04.2021 01:00


Mathematics, 30.04.2021 01:00

Engineering, 30.04.2021 01:00




Mathematics, 30.04.2021 01:00