
Chemistry, 23.08.2019 05:20 madelllinefreeman
Assume the hydrolysis of atp proceeds with δg′° = –30 kj/mol. atp + h2o → adp + pi which expression gives the ratio of adp to atp at equilibrium, if the [pi] = 1.0 m? (note: use rt = 2.5 kj/mol.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3


Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
Assume the hydrolysis of atp proceeds with δg′° = –30 kj/mol. atp + h2o → adp + pi which expression...
Questions in other subjects:





Biology, 14.10.2019 21:00



Mathematics, 14.10.2019 21:00


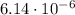
![K_{eq} = \frac{[ADP][Pi]}{ATP}](/tpl/images/0190/0516/0659d.png)
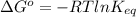
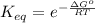
![\frac{[ADP]}{[ATP]} = \frac{[Pi]}{K_{eq}}](/tpl/images/0190/0516/65ede.png)
![\frac{[ADP]}{[ATP]} = \frac{[Pi]}{K_{eq}} = \frac{[Pi]}{e^{-\frac{\Delta G^o}{RT}}}](/tpl/images/0190/0516/83fab.png)
![\frac{[ADP]}{[ATP]} = \frac{[Pi]}{K_{eq}} = \frac{[Pi]}{e^{-\frac{\Delta G^o}{RT}}} = [Pi]e^{\frac{\Delta G^o}{RT}} = 1.0 M\cdot e^{\frac{-30 kJ/mol}{2.5 kJ/mol}} = 6.14\cdot 10^{-6}](/tpl/images/0190/0516/060d5.png)



