
Chemistry, 23.08.2019 04:30 elizabethburkha
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their respective ionis. for a 0.6 m concentration of cu2+, the lead electrode is oxidized yielding potential of 0.507 v. a cell a) calculate the concentration of pb2+ b) suppose the lead electrode is reduced, in that case what would be the concentration of pb2 what does this answer tell you?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

You know the right answer?
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their...
Questions in other subjects:

Mathematics, 01.12.2020 21:50

Chemistry, 01.12.2020 21:50




Mathematics, 01.12.2020 21:50


Mathematics, 01.12.2020 21:50


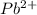 is, 0.0337 M
is, 0.0337 M
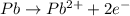
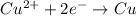
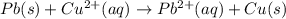
![E^o_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0189/9678/d356c.png)
![E^o_{[Cu^{2+}/Cu]}=+0.34V](/tpl/images/0189/9678/0d165.png)
![E^o=E^o_{[Cu^{2+}/Cu]}-E^o_{[Pb^{2+}/Pb]}](/tpl/images/0189/9678/2597c.png)
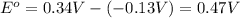
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Pb^{2+}]}{[Cu^{2+}]}](/tpl/images/0189/9678/2e714.png)
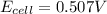
![0.507=0.47-\frac{0.0592}{2}\log \frac{[Pb^{2+}]}{(0.6)}](/tpl/images/0189/9678/33110.png)
![[Pb^{2+}]=0.0337M](/tpl/images/0189/9678/42065.png)

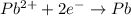

![E^o=E^o_{[Pb^{2+}/Pb]}-E^o_{[Cu^{2+}/Cu]}](/tpl/images/0189/9678/264d0.png)
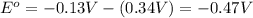
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Cu^{2+}]}{[Pb^{2+}]}](/tpl/images/0189/9678/9bec7.png)
![0.507=-0.47-\frac{0.0592}{2}\log \frac{(0.6)}{[Pb^{2+}]}](/tpl/images/0189/9678/15aa8.png)
![[Pb^{2+}]=6.093\times 10^{32}M](/tpl/images/0189/9678/e8ea8.png)


