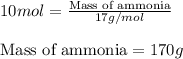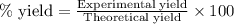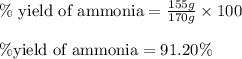Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g) + 3h2 (g) 2nh3 (g). if 140 grams of nitrogen gas is allowed to react with an excess of hydrogen gas to produce 155 grams of ammonia, what is the percent yield of this reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1



Chemistry, 23.06.2019 03:50, lindseyklewis1p56uvi
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g...
Questions in other subjects:


Mathematics, 09.03.2021 06:20

Mathematics, 09.03.2021 06:20

Mathematics, 09.03.2021 06:20

Mathematics, 09.03.2021 06:20

Mathematics, 09.03.2021 06:20

Mathematics, 09.03.2021 06:20


Mathematics, 09.03.2021 06:20


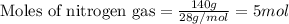
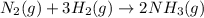
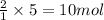 of ammonia.
of ammonia.