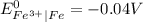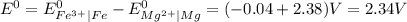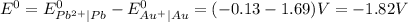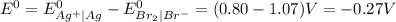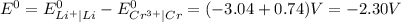Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
Determine which of the following pairs of reactants will result in a spontaneous reaction at 25°c. n...
Questions in other subjects:

Mathematics, 13.10.2019 19:00

History, 13.10.2019 19:00

Mathematics, 13.10.2019 19:00



Mathematics, 13.10.2019 19:00

Advanced Placement (AP), 13.10.2019 19:00


Mathematics, 13.10.2019 19:00

Mathematics, 13.10.2019 19:00

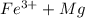 gives spontaneous reaction.
gives spontaneous reaction.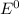 ) of the reaction is positive. Because it leads to negative standard gibbs free energy change (
) of the reaction is positive. Because it leads to negative standard gibbs free energy change (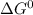 ), which is a thermodynamic condition for spontaneity of a reaction.
), which is a thermodynamic condition for spontaneity of a reaction.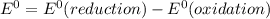
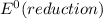 and
and 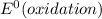 represents standard reduction potential of reduction half cell and standard reduction potential of oxidation half cell.
represents standard reduction potential of reduction half cell and standard reduction potential of oxidation half cell.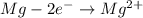 ;
; 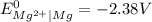
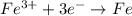 ;
;