
Chemistry, 22.08.2019 22:30 emmaraeschool
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol of pcl3 is added to 0.70 mol of cl2 in a 1.00-l reaction vessel at 230°c, what is the concentration of pcl3 when equilibrium has been established? a) 0.049 mb) 0.11 mc) 0.59 md) 0.30 me) 0.83 m

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1


You know the right answer?
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol...
Questions in other subjects:

Social Studies, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

English, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

History, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

Social Studies, 10.09.2020 22:01

Mathematics, 10.09.2020 22:01

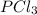 and
and 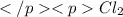 .
.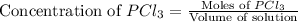
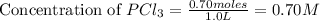
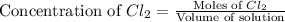
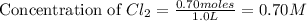
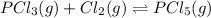
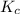 will be,
will be,![K_c=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0189/1195/4c8d0.png)
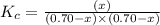
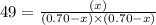
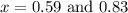
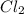 at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M
at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M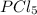 at equilibrium = x = 0.59 M
at equilibrium = x = 0.59 M

