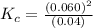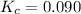Chemistry, 22.08.2019 18:30 bjpvrpow74wq
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350 mol of o2 and excess carbon were placed in a 5.00-l container and heated, the equilibrium concentration of co was found to be 0.060 m. what is the equilibrium constant, kc, for this reaction?
a. 0.001
b. 0.072
c. 0.090
d. 1.2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350...
Questions in other subjects:




History, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00





 = 0.350 mole
= 0.350 mole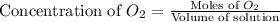
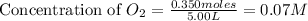
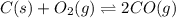
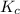 will be,
will be,![K_c=\frac{[CO]^2}{[O_2]}](/tpl/images/0188/5908/f4b67.png)
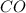 at equilibrium is, 0.060 M
at equilibrium is, 0.060 M