
Chemistry, 22.08.2019 18:10 ggdvj9gggsc
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of the following changes would result in equilibrium shifting towards the products. i. increase h2 ii. decrease o2 iii. add a catalyst iv. increase the temperature v. increase h2o2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of...
Questions in other subjects:




Biology, 02.11.2019 04:31


History, 02.11.2019 04:31



Mathematics, 02.11.2019 04:31

Mathematics, 02.11.2019 04:31

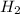 and
and  and reactant is
and reactant is 


