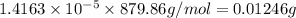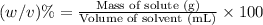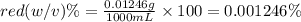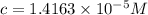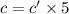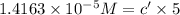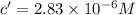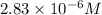Chemistry, 22.08.2019 17:20 kodakcam02
If the molar absorptivity constant for the red dye solution is 5.56×104 m-1cm-1, calculate the molarity of the red dye solution at the optimal wavelength 519nm and absorbance value 0.945
you may assume l = 1.20cm. hint: a=εlc
b. convert the molarity in part a to w/v%. show your work. molar mass of fd& c red #3 = 879.86g/mol refer to examples on next page
c. if you want to dilute the red dye solution in part a by 5 times in a single dilution step, explain in two sentences on how one should proceed with the dilution. in addition, calculate the final concentration of the diluted solution. show your work for the numerical part of this question.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, cefindley14
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2


You know the right answer?
If the molar absorptivity constant for the red dye solution is 5.56×104 m-1cm-1, calculate the molar...
Questions in other subjects:


English, 13.02.2020 19:56

Physics, 13.02.2020 19:56


Mathematics, 13.02.2020 19:56


Mathematics, 13.02.2020 19:56



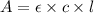
 = molar absorptivity of this solution =
= molar absorptivity of this solution =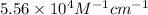
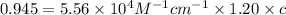
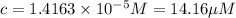
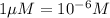 )
)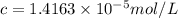
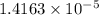 moles of red dye.
moles of red dye.