Chemistry, 22.08.2019 17:10 zoeyandblaze
Write the expression for the equilibrium constant kp for the following reaction. enclose pressures in parentheses and do not write the chemical formula as a subscript. for example, enter (pnh3)2 as (p nh3)2 .if either the numerator or denominator is 1, enter 1nh4hs(s) ↔ nh3(g) + h2s(g)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
Write the expression for the equilibrium constant kp for the following reaction. enclose pressures i...
Questions in other subjects:



Mathematics, 22.07.2019 18:00




English, 22.07.2019 18:00

Mathematics, 22.07.2019 18:00


History, 22.07.2019 18:00

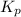 is written below.
is written below.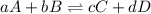
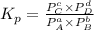
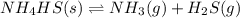
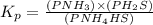
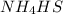 will be 1 because it is solid.
will be 1 because it is solid.