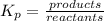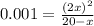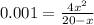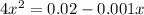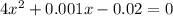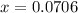Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the...

Chemistry, 22.08.2019 05:10 chrisraptorofficial
Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the expression for the equilibrium constant of this reaction.
if the initial concentration of a is 20 atm pressure, the initial concentration of b is 0 atm and the equilibrium constant kp for the reaction is .001 atm-1, calculate the equilibrium concentration of b.
i know the first part of this would be kc = [a] / [b]2 i need the second part

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
Questions in other subjects:

English, 05.01.2021 21:40

Social Studies, 05.01.2021 21:40


Mathematics, 05.01.2021 21:40

Mathematics, 05.01.2021 21:40



Mathematics, 05.01.2021 21:40

English, 05.01.2021 21:40

Social Studies, 05.01.2021 21:40

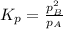 and equilibrium concentration of B is 0.141 atm.
and equilibrium concentration of B is 0.141 atm.