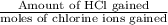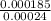Chemistry, 22.08.2019 03:30 shymitch32
Ahittorf cell fitted with ag/agcl electrodes is filled with 0.0106 molal aqueous hcl (molar mass: 36.458). a 2.00 ma current was passed for 10800 sec. the cathode solution was then found to weigh 51.7436 g and contain 0.0267 g hcl after analysis. what is the transport number of h+?
a. 0.177
b. 0355
c. 0.645
d. 0.823

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1


Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Ahittorf cell fitted with ag/agcl electrodes is filled with 0.0106 molal aqueous hcl (molar mass: 3...
Questions in other subjects:

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Health, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Physics, 18.03.2021 01:10


English, 18.03.2021 01:10

English, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

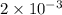 A
A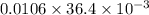
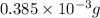
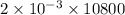 = 21.6 C
= 21.6 C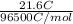
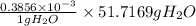
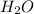 = 0.0267 g
= 0.0267 g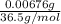
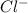 in cathodic compartment = 0.000224 mol
in cathodic compartment = 0.000224 mol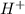 =
=