
Chemistry, 22.08.2019 02:20 lisacarter0804
The boiling point of bromine is 59 °c. which of the following best predicts the boiling point of iodine monochloride, a polar compound?
higher than 59 °c because dipole-dipole interactions in iodine monochloride are stronger than dispersion forces in bromine.
lower than 59 °c because ionic bonding in bromine is stronger than covalent bonding in iodine monochloride.
lower than 59 °c because dipole-dipole interactions in iodine monochloride are weaker than in bromine.
higher than 59 °c because ionic bonding in iodine monochloride is stronger than h-bonding in bromine.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 21.06.2019 20:10, maribel2421
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
You know the right answer?
The boiling point of bromine is 59 °c. which of the following best predicts the boiling point of iod...
Questions in other subjects:

Spanish, 02.07.2019 20:30



History, 02.07.2019 20:30

Mathematics, 02.07.2019 20:30


Mathematics, 02.07.2019 20:30


Health, 02.07.2019 20:30

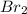 ) is a non-polar molecule due to the absence of a permanent dipole. The only force of attraction in non-polar molecules are the weak dispersion forces.
) is a non-polar molecule due to the absence of a permanent dipole. The only force of attraction in non-polar molecules are the weak dispersion forces. 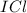 ) is a polar molecule which are held together dipole-dipole forces that are stronger than the London dispersion forces.
) is a polar molecule which are held together dipole-dipole forces that are stronger than the London dispersion forces. 

