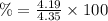Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
The solubility of a certain compound in ice-cold water is 0.16 g in 100 ml. its solubility in hot wa...
Questions in other subjects:


Health, 21.10.2019 15:00

Biology, 21.10.2019 15:00

English, 21.10.2019 15:00

Mathematics, 21.10.2019 15:00

History, 21.10.2019 15:00