
Chemistry, 21.08.2019 18:20 sparrgrovekyle
Amixture containing kclo3,k2co3,khco3, and kcl was heated, producing co2,o2, and h2o gases according to the following equations: 2kclo3(s)? 2kcl(s)+3o2(g)2khco3(s)? k2o(s)+h2o(g)+2co2(g)k2co3(s)? k2o(s)+co2(g)the kcl does not react under the conditions of the reaction. 100.0 g of the mixture produces 1.90 g of h2o, 13.64 of co2, and 4.10 g of o2. (assume complete decomposition of the mixture.)1. how many grams of kclo3 were in the original mixture? 2.how many grams of khco3 were in the original mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, duracohack
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1



Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1
You know the right answer?
Amixture containing kclo3,k2co3,khco3, and kcl was heated, producing co2,o2, and h2o gases according...
Questions in other subjects:

Mathematics, 02.11.2020 06:40


Mathematics, 02.11.2020 06:40

History, 02.11.2020 06:40

Mathematics, 02.11.2020 06:40


Advanced Placement (AP), 02.11.2020 06:40


Mathematics, 02.11.2020 06:40

Mathematics, 02.11.2020 06:40

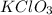 in the original mixture is 10.46 g
in the original mixture is 10.46 g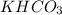 in the original mixture is 21.11 g
in the original mixture is 21.11 g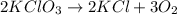
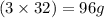 of oxygen is produced when
of oxygen is produced when 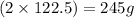 of potassium chlorate is decomposed.
of potassium chlorate is decomposed.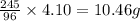 of potassium chlorate is decomposed.
of potassium chlorate is decomposed.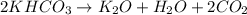
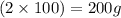 of potassium bicarbonate is decomposed.
of potassium bicarbonate is decomposed.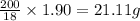 of potassium bicarbonate is decomposed.
of potassium bicarbonate is decomposed.

