Chemistry, 21.08.2019 18:20 allieballey0727
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed that the pressure of the gas in the tank is p, the volume of the tank is v and temperature is t. the ideal gas law can be applied. if the volume v and temperature t are constant, find how the pressure varies with time.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed th...
Questions in other subjects:





Chemistry, 07.12.2020 22:20

History, 07.12.2020 22:20


Health, 07.12.2020 22:20


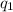
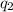
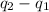 =
= 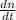 ....... (1)
....... (1)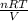
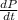 =
= 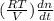
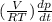
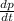 =
=