
Liquid water at 83 c and at 1 atm flows through a heated pipe at a flow rate of 3.9 kg/s. it then leaves the pipe as steam. the water receives 12378600 j/s from the pipe. calculate the temperature of the steam leaving the pipe. the water boiling point at the pressure of the system is 100 c. thermal properties: co of liquid water: 4200 j/kg. k cp of steam: 1800 j/kg. k enthalpy of evaporation of water: 276 j/kg

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Liquid water at 83 c and at 1 atm flows through a heated pipe at a flow rate of 3.9 kg/s. it then le...
Questions in other subjects:

Mathematics, 20.06.2020 00:57

Chemistry, 20.06.2020 00:57





Social Studies, 20.06.2020 00:57

History, 20.06.2020 00:57


Mathematics, 20.06.2020 00:57

 = (83 + 273) K = 356 K
= (83 + 273) K = 356 K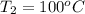 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K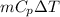
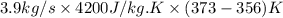
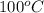 is as follows.
is as follows.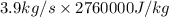

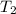 = 1552.83 K
= 1552.83 K

