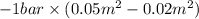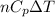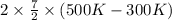Chemistry, 21.08.2019 02:20 BreBreDoeCCx
By heating 2 mol of nitrogen gas in a frictionless piston-cylinder, the gas expands at a constant pres- sure of 1 bar from an initial volume of 0.02 m² to a final volume of 0.05 m. the gas temperature correspondingly increases from 300 to 500 k. assuming nitrogen is an ideal gas with a molar heat capacity cp = r (where r is the gas constant), find the amount of heat added and work derived from the gas expansion.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
By heating 2 mol of nitrogen gas in a frictionless piston-cylinder, the gas expands at a constant pr...
Questions in other subjects:



Mathematics, 08.01.2020 04:31


Mathematics, 08.01.2020 04:31

Mathematics, 08.01.2020 04:31


History, 08.01.2020 04:31


Biology, 08.01.2020 04:31

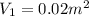 ,
, 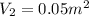
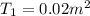 = 300 K ,
= 300 K , 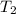 = 500 K
= 500 K